DiscoPlot allows the user to quickly identify genomic rearrangements, misassemblies and sequencing artefacts by providing a scalable method for visualising large sections of the genome. It reads single-end or paired read alignments in SAM, BAM or standard BLAST tab format and creates a scatter plot of opaque crosses representing the alignments to a reference. DiscoPlot is freely available (under a GPL license) for download (Mac OS X, Unix and Windows) at: https://github.com/BeatsonLab-MicrobialGenomics/DiscoPlot/releases.
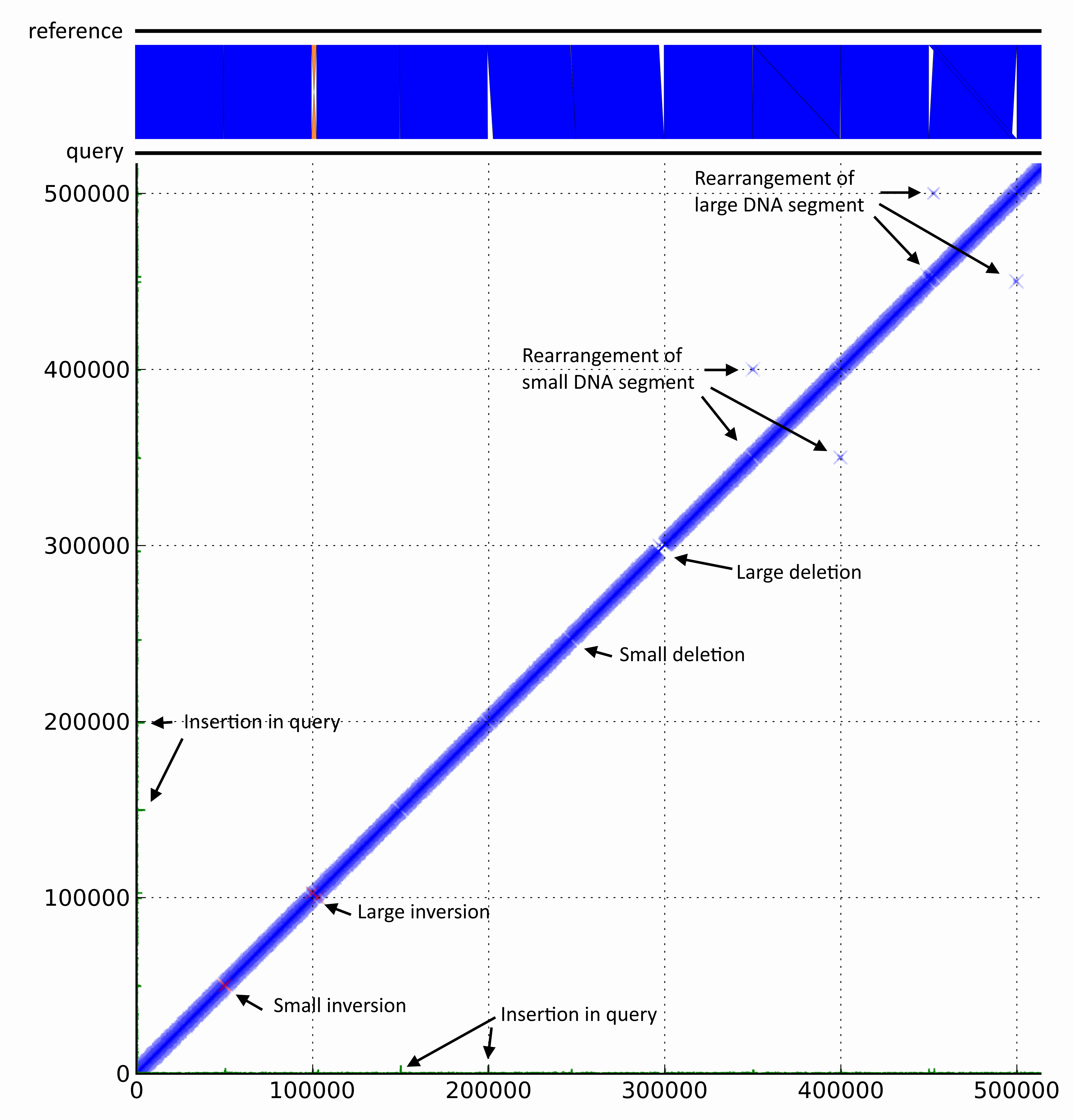
DiscoPlot of a mock genome. A mock genome was created by adding genomic rearrangements to the chromosome of E. coli str. UTI89. Paired-end reads generated from the mock genome (query) with GemSim and mapped back to UTI89 (reference). The first ~500 Kbp were then visualised using DiscoPlot.
Table of Contents
Please use this README.rst as the core DiscoPlot user documentation.
Cite this Github repository if you use DiscoPlot to generate figures for publications:
SULLIVAN MJ & BEATSON SA^. DiscoPlot - Visualising discordant reads. https://github.com/BeatsonLab-MicrobialGenomics/DiscoPlot.
- On the roadmap:
- Print selected read names, alignments or sequences
DiscoPlot is a commandline application. If you're not familiar with the commandline we recommend you ask local IT support to help you install it.
- You will need to install/have installed:
- python >= 2.7 (Python 3 is not supported)
- To automatically generate BLAST aligments (For long read DiscoPlots) using DiscoPlot you will need to install/have installed:
- ncbiblast+ >= 2.2.27
You can check these are installed by:
$ python --version $ blastn -version
Installation of python or blastn (without a package manager) is beyond the scope of this document.
If you have both python and blastn you need to (if not already present) install pip.
You can check if pip exists with:
$ which pip
If you get a "not found", please read the pip installation instructions.
If you already have pip we do suggest you upgrade it. We are using version 1.5.6 at the time of writing this document.
You can upgrade pip like this:
$ pip install --upgrade pip
The following python libraries should be installed (automatically) if you follow the installation instructions detailed below.
- We use the following python libraries:
- numpy >= 1.8.1
- matplotlib >= 1.3.1
- pysam >= 0.8.1
Pysam is only required for generating DiscoPlots with BAM files. SAM compatability has been included to allow windows users to generate DiscoPlots. PySam will not install on Windows, don't bother trying (or if you've succeeded please let me know how).
Discoplot uses 3rd party packages that are extremely important for scientific computing but may be difficult to install. While pip install * *--user DiscoPlot may work we recommend you install these 3rd party packages using apt-get.
Run:
$ sudo apt-get install python-numpy python-matplotlib
Now pip install DiscoPlot:
$ pip install --user DiscoPlot
We use the --user option of pip to put DiscoPlot in: /home/$USER/.local/bin/ You need to add this location to you ~/.bash_profile.
Add DiscoPlot to your path:
$ echo 'export PATH=$PATH:/home/$USER/.local/bin/' >> ~/.bash_profile
Finally install BLAST+:
$ sudo apt-get install ncbi-blast+
You'll need to have the equivalents of python-dev libatlas-dev liblapack-dev gfortran libfreetype6-dev libfreetype6 & libpng-dev installed. We had no problems installing DiscoPlot on a recently acquired OSX Mavericks machine using the homebrew package manager.
The installed packages on this machine via:
$ brew list
Are available at this gist.
pip install DiscoPlot:
$ pip install --user DiscoPlot
We use the --user option of pip to put DiscoPlot in: /Users/$HOME/Library/Python/2.7/bin You need to add this location to you ~/.bash_profile.
Add DiscoPlot to your path:
$ echo 'export PATH=$PATH:/Users/$HOME/Library/Python/2.7/bin/ >> ~/.bash_profile'
Finally install BLAST+:
$ sudo brew install blast
Download and install numpy and matplotlib. To make this process easier you can download a distribution of python with matplotlib and numpy already installed such as anaconda.
pip install DiscoPlot:
$ pip install DiscoPlot
Finally download and install BLAST.
Run:
$ DiscoPlot -h $ python -c 'import DiscoPlot; print DiscoPlot'
You can upgrade like this:
pip install --upgrade DiscoPlot
Please regularly check back to make sure you're running the most recent DiscoPlot version.

DiscoPlot of a mock genome. A mock genome was created by adding genomic rearrangements to the chromosome of E. coli str. UTI89. Paired-end reads generated from the mock genome (query) with GemSim (ref) and mapped back to UTI89 (reference). The first ~500 Kbp were then visualised using DiscoPlot.
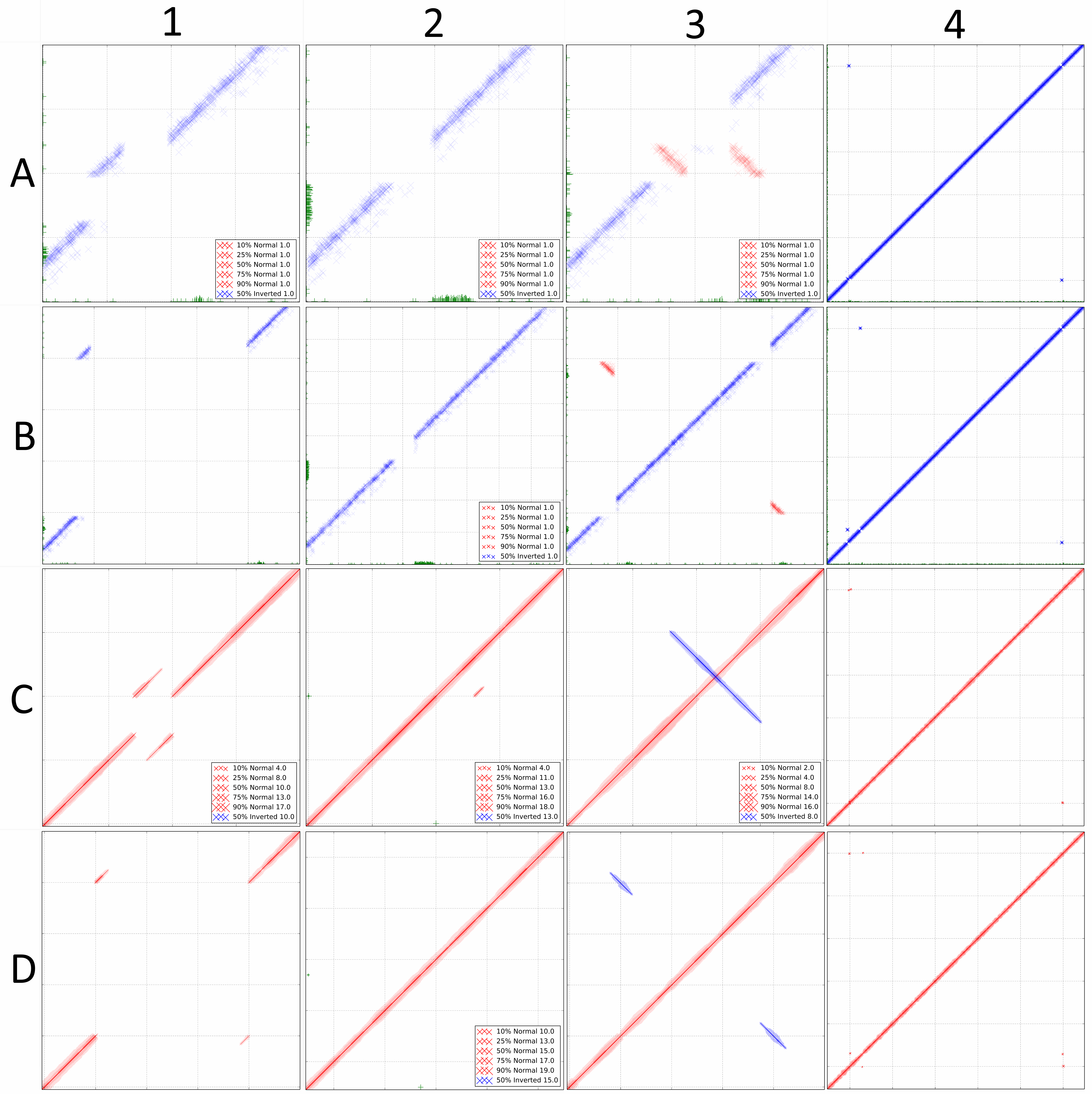
DiscoPlots of common structural variants. Each box shows a common genomic rearrangement represented by a DiscoPlot. Rows A and B were created using 100 bp long paired-end reads with an insert size of 300bp. Rows C and D were created using single-end reads with an average length of 1000bp. For each box the rearrangement in the sequenced genome is listed, followed by the scale of the gridlines in brackets. A1, C1: 300 bp deletion (400 bp). A2, C2: 300 bp insertion (400 bp). A3, C3: 300 bp inversion (400 bp). A4, C4: 300 bp sequence translocated 50 Kbp upstream (10 Kbp). B1, D1: 3000 bp deletion (1000 bp). B2, D2: 3000 bp insertion (500 bp). B3, D3: 3000 bp inversion (1000 bp). B4, D4: 3000 bp sequence translocated 50 Kbp upstream (10 Kbp).
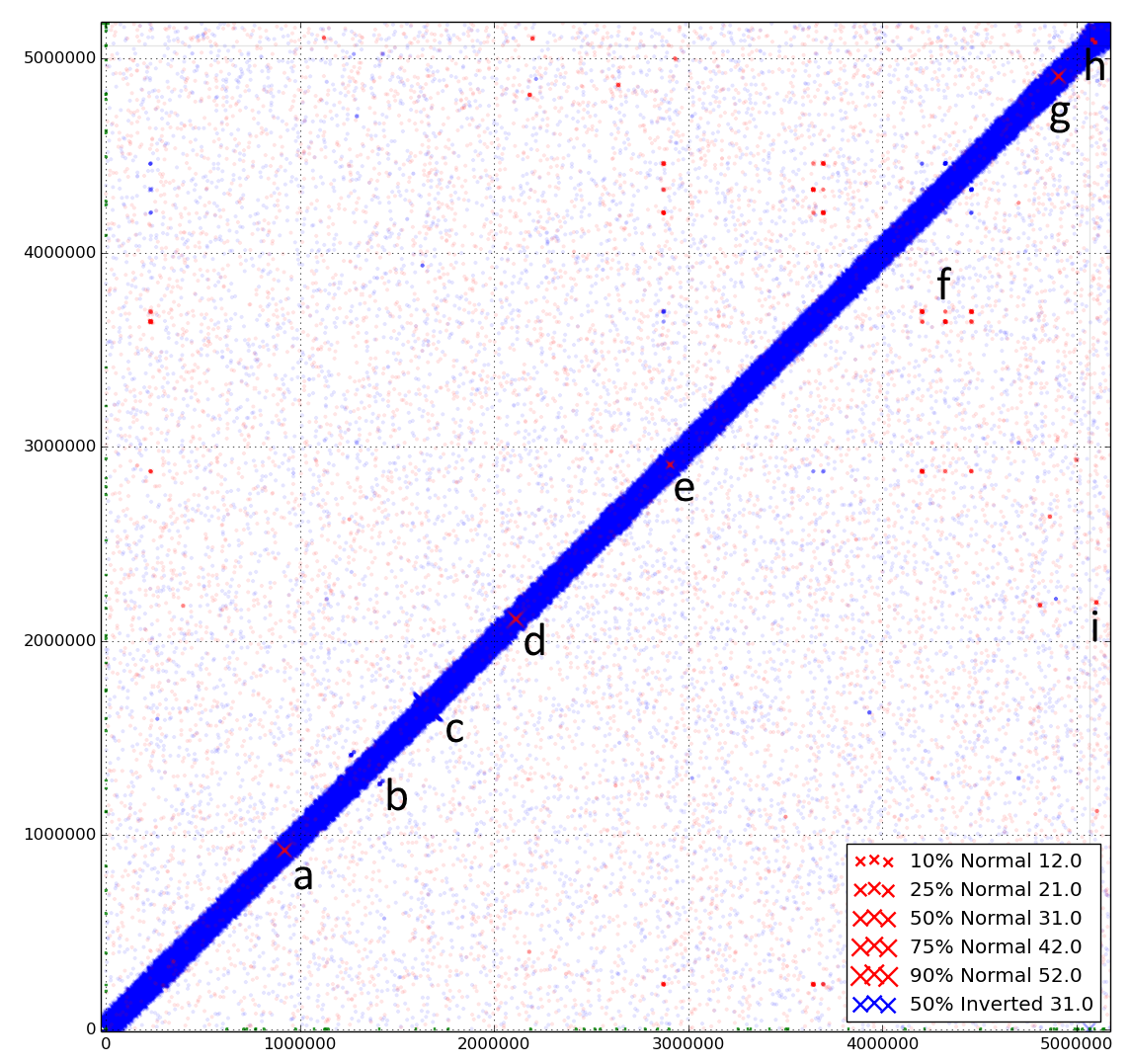
The dynamic nature of the genome of Escherichia coli str. UTI89. Discoplot of paired-end reads from a clonal culture of UTI89 mapped back to the published reference chromosome and plasmid. Coordinates from 0 to 5,065,741 represent the chromosome of E. coli UTI89, coordinates ≥ 5,066,000 represent the plasmid of E. coli UTI89
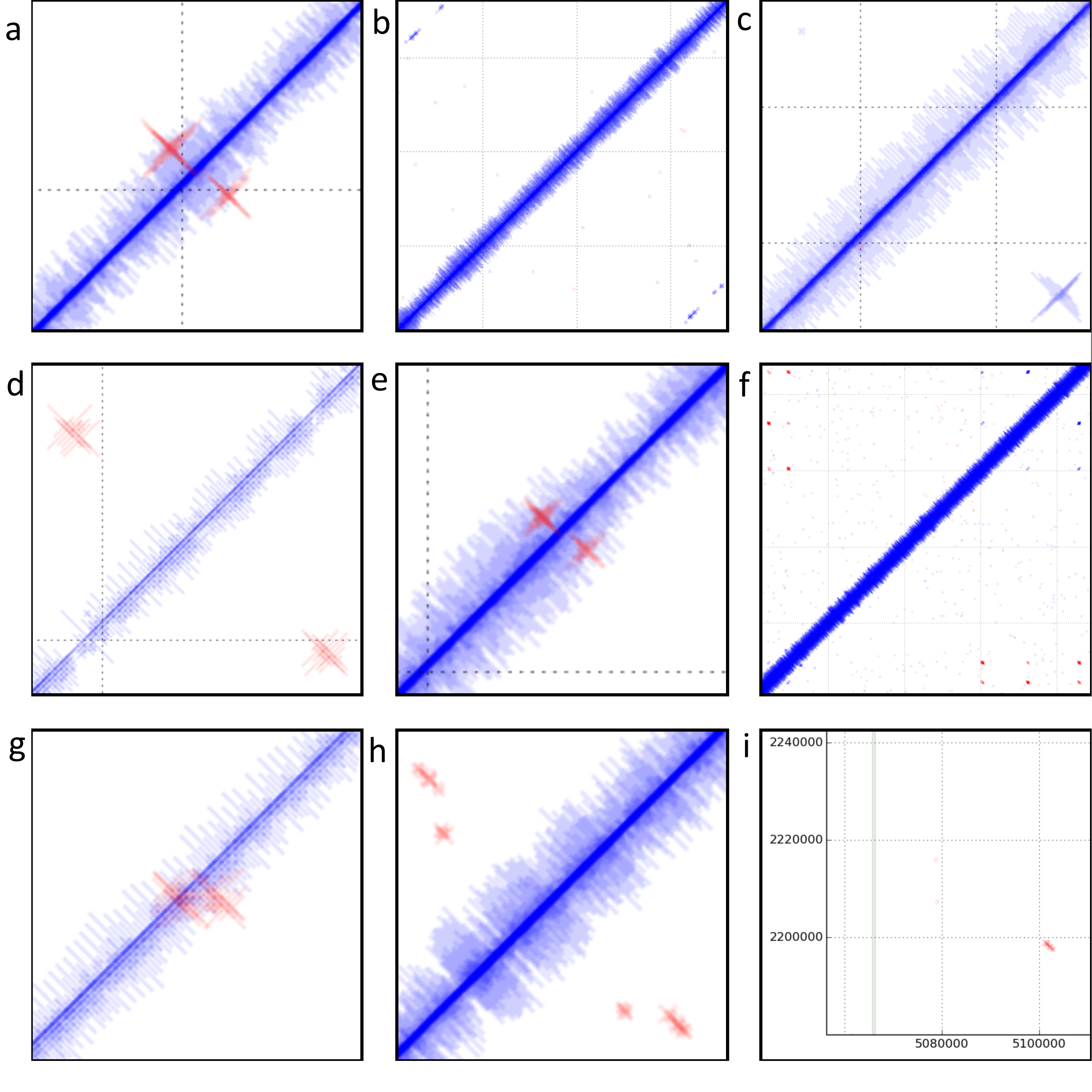
Discordant reads in E. coli str. UTI89. a) Read alignment indicates inversion of bases 919,638..922,323. 12bp inverted repeat present at terminals of region. Start and stop of inverted region occurs in two probable tail fibre proteins. Two additional tail fibre assembly proteins are encoded within the boundaries of this region. Region is immediately downstream of a putative DNA invertase gene. b, f, h, i) Reads are misaligned as they map equally well in a concordant position. c) Read alignment indicates circularisation of bases 1,653,000..1,662,603. 17bp direct repeats present at terminals of this region. Region also encodes five putative phage-related membrane proteins, two putative phage proteins, three phage hypothetical proteins, four hypothetical proteins and a single putative phage related secreted protein. Size of crosses indicates coverage of this region is higher than average. Only a single read (indicated by the cross, top left) indicates potential excision of this region. d) Read alignments indicate inversion of bases 2,109,690..2,114,003. Region contains ~100bp inverted repeat at terminals which encodes a tRNA. Region contains 3 hypothetical proteins and an additional tRNA identical to the repeats. A P4-phage integrase is present immediately downstream of the inversion. The lack of concordantly mapping reads at prophage boundary indicates that the inverted phage has reached fixation in the population. e) Reads indicate inversion of bases 2,906,008..2,906,936. 15bp inverted repeats present at terminals of this region. The 3’ end of a putative tail fibre assembly gene is encoded by this region. g) Read alignments indicate inversion of bases 4,907,424..4,907,737. Regions has 9bp inverted repeat at terminals. It is located in a non-coding region between fimA and fimE which encode the type I fimbriae.
Align reads with your favourite short read aligner (e.g. BWA, Bowtie2)
Create DiscoPlot from sam file with 5000 bins - open in a matplotlib window:
DiscoPlot.py -sam sam_file.sam -s 5000
Create a DiscoPlot from a bam file using a bin size of 10,000bp - save as .png file:
DisocPlot.py -bam bam_file.bam -bin 10000 -o discoplot.png
To automatically generate a BLAST alignment using BLAST+ run:
DiscoPlot.py -r reads.fa -ref reference.fasta -B -s 5000
To provide DiscoPlot with an alignment file (BLAST tab delimited format):
DiscoPlot -ref reference.fasta -b alignment.out -s 5000
More Coming Soon
DiscoPlot.py - Visualising discordant reads.
In paired read mode DiscoPlot must be provided with a BAM or SAM file. In Single read mode DiscoPlit must be provided with a alignment file (BLAST tab delimited format) or reads and a reference (in FASTA format).
-bin (size of bins in bp) or -s (size of bins) must be specified.
additional arguments:
-h, --help show this help message and exit
-r READ_FILE, --read_file READ_FILE
read file - provide DiscoPlot with a read file to
BLAST (long read mode).
-ref REFERENCE_FILE, --reference_file REFERENCE_FILE
Reference file - Reference for generating long reads
alignments.
-bam BAM_FILE, --bam_file BAM_FILE
bam file - paired read mode. (Requires pysam).
-sam SAM_FILE, --sam_file SAM_FILE
sam file - paired read mode. (pysam not required)
-hm HEATMAP, --heatmap HEATMAP
Heatmap file - provide DiscoPlot with custom generated
heatmap.
-B GEN_BLAST, --gen_blast GEN_BLAST
Generate blast files, use argument as prefix for
output.
-b BLAST_FILE, --blast_file BLAST_FILE
Provide DiscoPlot with alignment file (long read mode)
(BLAST tab delimited file - output format 6)
-o OUTPUT_FILE, --output_file OUTPUT_FILE
output file [gif/bmp/png]
-s SIZE, --size SIZE Number of bins.
-bin BIN_SIZE, --bin_size BIN_SIZE
Bin size (in bp)
-g GAP, --gap GAP Gap size - gap size between entries in reference.
-sub SUBSECTION [SUBSECTION ...], --subsection SUBSECTION [SUBSECTION ...]
Only display subection of genome [ref]/[min_cutoff
max_cutoff]/[ref min_cutoff max_cutoff]
-wb WRITE_READS [WRITE_READS ...], --write_reads WRITE_READS [WRITE_READS ...]
Write reads in rectangle to bam/sam [x1 y1 x2 y2
out.bam]
-c MIN_HITS, --min_hits MIN_HITS
Only show bins with more than this number of hits.
-m MAX_HITS, --max_hits MAX_HITS
Only show bins with less hits than this.
-dpi IMAGE_QUALITY, --image_quality IMAGE_QUALITY
Image quality (in DPI)
-i MIN_IDENT, --min_ident MIN_IDENT
Min. idenity of hits to draw (long read mode).
-l MIN_LENGTH, --min_length MIN_LENGTH
Min. length of hits to draw (long read mode).
-d UNMAPPED, --unmapped UNMAPPED
Unmapped bases on edge for RMaD to consider read
partially unmapped.
-a ALPHA, --alpha ALPHA
Transparency of mapped read markers
-a2 ALPHA2, --alpha2 ALPHA2
Transparency of unmapped read markers
-mc M_COUNT, --m_count M_COUNT
The count of a bin to be used as the median value for
calculating the size of the dot [auto]
-ms M_SIZE, --m_size M_SIZE
Set the width (in bins) of a marker with a median
count.
-log, --log Log10 bin counts. (For data with highly variable
coverage).
-sw, --switch Draw most common (inverted/direct) hits first.
-nl, --no_legend Don't create legend.
-ng, --no_gridlines Don't draw gridlines.
-na, --no_label No axis labels.
-split SPLIT_GRAPH [SPLIT_GRAPH ...], --split_graph SPLIT_GRAPH [SPLIT_GRAPH ...]
Show multiple subsections of graph [start1 stop1
start2 stop2 etc.] or [ref1 start1 stop1 ref2 start2
stop2 etc.]
-hl HIGHLIGHT [HIGHLIGHT ...], --highlight HIGHLIGHT [HIGHLIGHT ...]
Highlight subsections of graph [alpha start1 stop1
start2 stop2 etc.] or [alpha ref1 start1 stop1 ref2
start2 stop2 etc.]
-mw MARKER_EDGE_WIDTH, --marker_edge_width MARKER_EDGE_WIDTH
Marker width (default is roughly 20x bin size)
Thanks for using DiscoPlot.py